Answer:
The total number of Cl atoms in 150mL of liquid CCl4 is 3.73*10²⁴.
Step-by-step explanation:
First you must determine the mass of CCL4 present in 150mL of CCl4. Density is a quantity that allows us to measure the amount of mass in a certain volume of a substance, whose expression for its calculation is the quotient between the mass of a body and the volume it occupies:
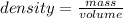
In this case, the density value of d = 1.589 g/mL. Then, being the volume equal to 150 mL, the value of the mass can be calculated as:
mass= density*volume
mass=1.589 g/mL * 150 mL
mass= 238.35 g
Now, being the molar mass of CCl4 154 g/mol, the number of moles that 238.35 g represents is calculated as:
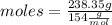
moles= 1.55
1 mole of the compound CCl4 contains 4 moles of Cl. Then, using a simple rule of three, it is possible to calculate the number of moles of Cl that 1.55 moles of CCl4 contain:
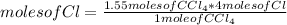
moles of Cl= 6.2
Avogadro's Number or Avogadro's Constant is called the number of particles that make up a substance (usually atoms or molecules) and that can be found in the amount of one mole of said substance. Its value is 6.023*10²³ particles per mole. Avogadro's number applies to any substance. In this case it can be applied as follows: if 1 mole of Cl contains 6.023*10²³ atoms, 6.2 moles of Cl how many atoms does it contain?
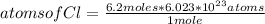
atoms of Cl= 3.73*10²⁴
The total number of Cl atoms in 150mL of liquid CCl4 is 3.73*10²⁴.