Answer:
45.78 g NaClO
Step-by-step explanation:
The reaction that takes place is:
- 2NaOH + Cl₂ → NaCl + NaClO + H₂O
In order to react completely, 1.36 moles of chlorine would require (2*1.36) 2.72 moles of NaOH. There are more moles than that, so NaOH is the limiting reactant.
We calculate the moles of NaClO formed, from the limiting reactant:
- 1.23 mol NaOH *
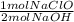 = 0.615 mol NaClO
= 0.615 mol NaClO
Finally we convert NaClO moles to grams, using its molecular weight:
- 0.615 mol NaClO * 74.44 g/mol = 45.78 g NaClO