Answer:
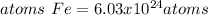
Step-by-step explanation:
Hello!
In this case, since the mass of iron (III) oxide is missing we use 80.0 g as found on similar problems. In such a way, since iron (III) molar mass is 159.70 g/mol, one mole of iron (III) oxide has two moles of iron (subscript) and one mole of atoms of iron have 6.022x10²³ atoms (Avogadro's number), the correct number of atoms in such sample is:
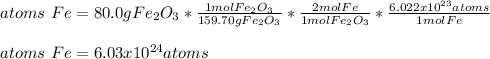
Which is shown with three significant figures as 80.0 g has three as well.
Best regards!