Answer:
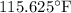
Step-by-step explanation:
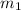 = First mass of water = 12 oz
= First mass of water = 12 oz
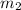 = Second mass of water = 20 oz
= Second mass of water = 20 oz
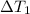 = Temperature difference of the solution with respect to the first mass of water =
= Temperature difference of the solution with respect to the first mass of water =
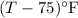
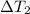 = Temperature difference of the solution with respect to the second mass of water =
= Temperature difference of the solution with respect to the second mass of water =
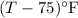
c = Specific heat of water
As heat gain and loss in the system is equal we have
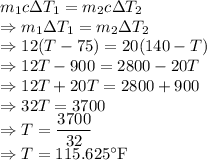
The final temperature of the solution is
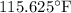 .
.