Answer:
The density of the unknown substance is 0.75 g/mL.
Step-by-step explanation:
We are given the volume and mass of a substance and are asked to find the density.
Density is determined with the formula
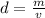 , where d is the density, m is the mass of the substance, and v is the volume the substance occupies.
, where d is the density, m is the mass of the substance, and v is the volume the substance occupies.
Therefore, the density can be found by dividing the mass and volume.
We are given and can determine that:
- The volume is 120 mL (v).
- The mass of the substance is 90 grams (m).
- Our derived unit is going to be g/mL.
Now, let's set up the math required.
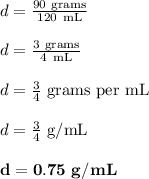
Therefore, the density of the substance is 0.75 g/mL.