Hey There!
_____________________________________
Answer:
![\huge\boxed{5.1x[tex]10^(14)]() Hz}[/tex]
Hz}[/tex]
_____________________________________
DATA:
E =
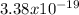
h =
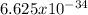
f = ?
_____________________________________
SOLUTION:
Energy of Photon is given by,
E = hf
Rearrange the equation,
f =
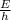
where,
E = energy
h = planck's constant
f = frequency
------------------------------------------------------------------------------------------------------------
Substitute the variable in the equation,
f =
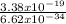
Simplify the equation,
f = 5.10 x 10^14 Hz
_____________________________________
Best Regards,
'Borz'