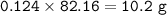Mass of Cyclohexen : 10.2 g
Further explanation
Reaction
3 C6H10 + 4 Na2Cr2O7 + 16 H2SO4 >
3 C6H10O4 + 4 Cr2(SO4)3 + 4Na2SO4 + 16 H2O
mol C6H10 : mol C6H10O4 = 3 : 3 = 1 : 1

12.5 grams of C6H10O4 , mol C6H10O4 (MW 146.14 g/mol):
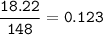
mol C6H10 = mol C6H10O4 = 0.124
mass C6H10 :