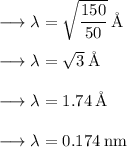Answer:
(c) 0.174 nm
Step-by-step explanation:
According to de Broglie hypothesis, the wavelength of the wave associated with electron is given by:
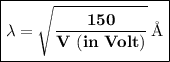
V → Potential Difference (50.0 V)
By substituting value of potential difference in the equation, we get: