Answer:
The mass of oxygen in liquid phase = 14.703 kg
The mass of oxygen in the vapor phase = 20.302 kg
Step-by-step explanation:
Given that:
The mass of the oxygen
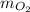 = 35 kg
= 35 kg
The mass of the nitrogen
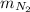 = 40 kg
= 40 kg
The cooling temperature of the mixture T = 84 K
The cooling pressure of the mixture P = 0.1 MPa
From the equilibrium diagram for te-phase mixture od oxygen-nitrogen as 0.1 MPa graph. The properties of liquid and vapor percentages are obtained.
i.e.
Liquid percentage of
 = 70% = 0.70
= 70% = 0.70
Vapor percentage of
 = 34% = 0.34
= 34% = 0.34
The molar mass (mm) of oxygen and nitrogen are 32 g/mol and 28 g/mol respectively
Thus, the number of moles of each component is:
number of moles of oxygen = 35/32
number of moles of oxygen = 1.0938 kmol
number of moles of nitrogen = 40/28
number of moles of nitrogen = 1.4286 kmol
Hence, the total no. of moles in the mixture is:
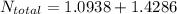
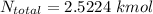
So, the total no of moles in the whole system is:
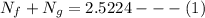
The total number of moles for oxygen in the system is
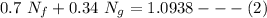
From equation (1), let N_f = 2.5224 - N_g, then replace the value of N_f into equation (2)
∴
0.7(2.5224 - N_g) + 0.34 N_g = 1.0938
1.76568 - 0.7 N_g + 0.34 N_g = 1.0938
1.76568 - 0.36 N_g = 1.0938
1.76568 - 1.0938 = 0.36 N_g
0.67188 = 0.36 N_g
N_g = 0.67188/0.36
N_g = 1.866
From equation (1)
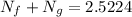
N_f + 1.866 = 2.5224
N_f = 2.5224 - 1.866
N_f = 0.6564
Thus, the mass of oxygen in the liquid and vapor phases is:
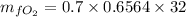
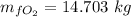
The mass of oxygen in liquid phase = 14.703 kg
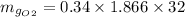
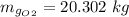
The mass of oxygen in the vapor phase = 20.302 kg