This tablet insufficient to neutralize the acid in the patient's stomach
Further explanation
Reaction
Al(OH)₃(s) + 3HCl(aq) ⇒ 3 H₂O(l) + AlCl₃(aq)
0.25 g of Aluminum hydroxide-Al(OH)₃ , mol (MW=78 g/mol) :
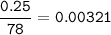
mol HCl : mol Al(OH)₃ = 3 : 1
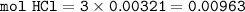
mass HCl (MW=36,46 g/mol) :
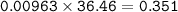
0.351 < 0.88⇒this tablet insufficient to neutralize the acid