Answer:
66.7%
Step-by-step explanation:
The reaction for the titration of the excess ferrous ion is:
- 5Fe⁺² + MnO₄⁻ + 8H⁺ → 5Fe³⁺ + Mn²⁺ + 4H₂O
We calculate the moles of Fe⁺² from the used moles of KMnO₄:
- 0.02 M * 15.0 mL = 0.30 mmol KMnO₄
- 0.3 mmol KMnO₄ *
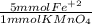 = 1.5 mmol Fe⁺²
= 1.5 mmol Fe⁺²
Then we substract those 0.30 mmol from the original amount used:
- 0.1 M * 50.0 mL = 5.0 mmol Fe⁺²
- 5.0 - 1.5 = 3.5 mmol Fe⁺²
The reaction between ferrous ammonium sulfate and MnO₂ is:
- 2Fe⁺² + MnO₂ + 4H⁺ → 2Fe³⁺ + Mn²⁺ + 2H₂O
So we convert those 3.5 mmol Fe⁺² that were used in this reaction to MnO₂ moles:
- 3.5 mmol Fe⁺² *
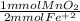 = 1.75 mmol MnO₂
= 1.75 mmol MnO₂
Then we convert MnO₂ to Mn₃O₄, using the reaction:
- 1.75 mmol MnO₂ *
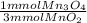 = 0.583 mmol Mn₃O₄
= 0.583 mmol Mn₃O₄
Finally we convert Mn₃O₄ moles to grams:
- 0.583 mmol Mn₃O₄ * 228.82 mg/mmol = 133.40 mg Mn₃O₄
And calculate the percent
- 133.40 / 200 * 100% = 66.7%