Answer:
a. 167 mL.
b. 39.3 %.
Step-by-step explanation:
Hello!
In this case, for the undergoing chemical reaction, since 45.0 g of aluminum react, based on the 2:3 mole ratio with sulfuric acid, we can compute the required moles as shown below:
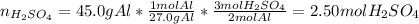
Next, since the molarity of a solution is computed based on the moles and volume (M=n/V), we can compute the required volume of sulfuric acid as shown below:
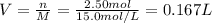
That in mL is 167 mL.
Moreover, for the percent yield, we compute the grams of aluminum sulfate that are produced based on the required 2.50 moles of sulfuric acid:
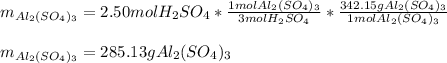
Therefore the percent yield is:

Best regards!