Answer:
19g
Step-by-step explanation:
Given:
Number of molecules = 1.505 x 10²³molecules
Formula of compound = CS₂
Unknown:
Mass of the compound
Solution:
A mole of every substance contains 6.02 x 10²³molecules. Using this we can find the number of moles from number of molecules given;
6.02 x 10²³molecules makes up 1 mole of a substance
1.505 x 10²³molecules will contain
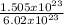 = 0.25mole of CS₂
= 0.25mole of CS₂
Mass of CS₂ = number of moles x molar mass
Molar mass of CS₂ = 12 + 2(32) = 76g/mol
Mass of CS₂ = 0.25 x 76 = 19g