Answer:
60.0 mL
General Formulas and Concepts:
Chemistry - Solutions
- Reading a Periodic Table
- Using Dimensional Analysis
- Molarity = moles of solute / liters of solution
Step-by-step explanation:
Step 1: Define
6.33 g CuNO₃
0.840 M Solution
Step 2: Identify Conversions
1 L = 1000 mL
Molar Mass of Cu - 63.55 g/mol
Molar Mass of N - 14.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of CuNO₃ - 63.55 + 14.01 + 3(16.00) = 125.56 g/mol
Step 3: Convert
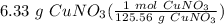 = 0.050414 mol CuNO₃
= 0.050414 mol CuNO₃
Step 4: Find volume
- Substitute: 0.840 M = 0.050414 mol / x L
- Multiply x on both sides: (x L)(0.840 M) = 0.050414 mol
- Divide M on both sides: x L = 0.060017
Step 5: Convert
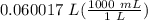 = 60.0168 mL
= 60.0168 mL
Step 6: Check
We are given 3 sig figs. Follow sig fig rules and round.
60.0168 mL ≈ 60.0 mL