Answer:

Step-by-step explanation:
We need to use the formula for heat of vaporization.
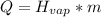
Identify the variables.
- The heat absorbed by the evaporating water is the latent heat of vaporization. For water, that is 2260 Joules per gram.
- Q is the energy, in this problem, 50,000 Joules.
- m is the mass, which is unknown.
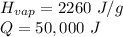
Substitute the values into the formula.
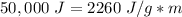
We want to find the mass. We must isolate the variable, m.
m is being multiplied by 2260 J/g. The inverse operation of multiplication is division. Divide both sides by 2260 J/g.
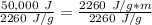
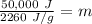
Divide. Note that the Joules (J) will cancel each other out.
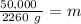
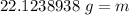
Round to the nearest whole number. The 1 in the tenth place tells us to leave the number as is.
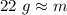
The mass is about 22 grams, so choice B is correct.