Answer:
The volume that a 200 g gold sample would have is 10.36 mL.
Explanation:
Density is defined as the property that matter, whether solid, liquid or gas, has to compress into a given space. In other words, density is a quantity that allows us to measure the amount of mass in a certain volume of a substance. Then, the expression for the calculation of density is the quotient between the mass of a body and the volume it occupies:
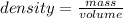
In this case it is known that the density of gold is 19.3 g / mL. This indicates that in 1 mL there are 19.3 g. Then the following rule of three can be applied: if 19.3 g is contained in 1 mL, how much volume is 200 g of gold?
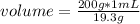
volume= 10.36 mL
The volume that a 200 g gold sample would have is 10.36 mL.