Answer:
8 kmol/s
Step-by-step explanation:
From the given information:
The combustion reaction equation for Octane in a stoichiometric condition can be expressed as:
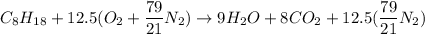
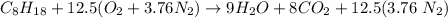
In the combustor, it is said that 60% of excess air and 1 mole of Octane is present.
Thus;
the air supplied = 1.6 × 12.5 = 20
The equation can now be re-written as:
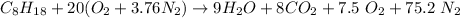 because for 1 mole of Octane, 8 moles of CO2 can be found in the combustion product.
because for 1 mole of Octane, 8 moles of CO2 can be found in the combustion product.
Thus, for 1 kmol/s of Octane also produce 8 kmol/s of CO2.
∴
The mole flow rate in Kmol/s of CO2 in the product stream = 8 kmol/s