Answer:
3.1atm
Step-by-step explanation:
Given parameters:
Volume of gas = 2L
Number of moles = 0.25mol
Temperature = 25°C = 25 + 273 = 298K
Unknown:
Pressure of the gas = ?
Solution:
To solve this problem, we use the ideal gas equation.
This is given as;
PV = nRT
P is the pressure
V is the volume
n is the number of moles
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
T is the temperature
P =
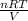
Now insert the parameters and solve;
P =
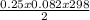 = 3.1atm
= 3.1atm