Answer:
0.354L
Step-by-step explanation:
Given parameters:
Molarity of KCN = 0.025M
Number of moles = 8.84 x 10⁻³mol
Unknown:
Volume of KCN = ?
Solution:
The molarity of a substance is expressed as;
Molarity =
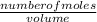
Volume of KCN =
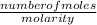
Now insert the parameters and solve;
Volume of KCN =
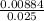 = 0.354L
= 0.354L