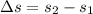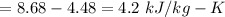Solution :
Given
Volume,
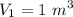
Temperature,
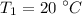
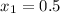
From the saturated water table, corresponding to
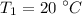 , we get the saturated liquid, vapor specific and the entropy.
, we get the saturated liquid, vapor specific and the entropy.
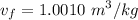
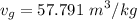
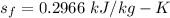
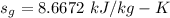
Now calculating the initial specific volume
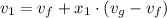
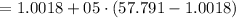
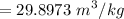
Calculating the initial specific entropy:
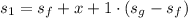
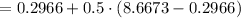
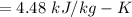
So final volume of the vessel is two times bigger as the initial volume
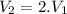
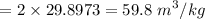
If we interpolate the values from tables between
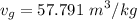 and
and
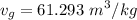 , we can get final temperature and specific entropy corresponding to value of
, we can get final temperature and specific entropy corresponding to value of
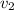 :
:
Final temperature,
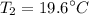
and
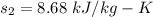
Calculating change in entropy