Answer:
1.02mole
Step-by-step explanation:
The reaction equation is given as:
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
Given:
Mass of H₂SO₄ = 50g
Unknown:
Number of moles of NaOH = ?
Solution:
To solve this problem, we first find the number of moles of the acid given;
Number of moles =

Molar mass of H₂SO₄ = 2(1) + 32 + 4(16) = 98g/mol
Now;
Number of moles =
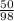 = 0.51mole
= 0.51mole
From the balanced reaction equation:
1 mole of H₂SO₄ will be neutralized by 2 mole of NaOH
0.51 mole of H₂SO₄ will be neutralized by 2 x 0.51 = 1.02mole of NaOH