Answer:
There must be added 500 cm3 of alcohol to bring the concentration to 80%.
Step-by-step explanation:
Concentration
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solution.
In the container, there are 2 liters (2000 cubic centimeters) of a solution, 75% of which is alcohol (solute) and 25% of water (solvent).
The original amount of alcohol is:
H = 2000*75% = 1500 cc
The original amount of water is:
W = 2000*25% = 500 cc
When we add x cc of pure alcohol, there are 1500+x cc of alcohol out of 2000+x cc of solution.
The new concentration is calculated as:
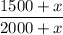
And it's known this concentration is 80% (0.8), thus:
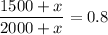
Multiplying by 2000+x:
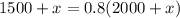
Operating:
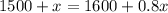
Rearranging:
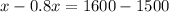
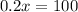
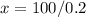
x= 500
There must be added 500 cm3 of alcohol to bring the concentration to 80%