Answer:
220.8L
Step-by-step explanation:
Given parameters:
Initial volume = 50L
Initial temperature = 15°C = 288K
Initial pressure = 640mmHg
Final temperature = 45°C = 318K
Final pressure = 160mmHg
Unknown:
Final volume = ?
Solution:
Using the combined law;
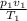 =
=
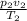
p,v and T are pressure, volume and Temperature
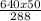 =
=
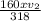
160 x 288 x v₂ = 640 x 50 x 318
v₂ = 220.8L