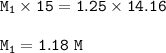The molarity of the unknown acid : 1.18 M
Further explanation
Titration is a procedure for determining the concentration of a solution by reacting with another solution that is known to be concentrated (usually a standard solution). Determination of the endpoint/equivalence point of the reaction can use indicators according to the appropriate pH range
Titration formula
M₁V₁n₁=M₂V₂n₂
monoprotic acid⇒n₁=1
NaOH monoprotic base⇒n₂=1