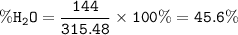In order of increasing percent water content:CoCl₂.6H₂O, Ba(OH)₂.8H₂O, MgSO₄.7H₂O
Further explanation
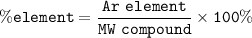
CoCl₂.6H₂O.MW=237.90 g/mol
6H₂O MW = 6.18=108 g/mol
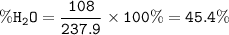
MgSO₄.7H₂O.MW=246.48 g/mol
MW 7H₂O = 7.18=126 g/mol
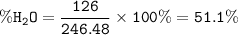
Ba(OH)₂.8H₂O MW=315.48 g/mol
MW 8H₂O = 8.18=144 g/mol