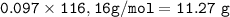Grams of ethyl butyrate produced : 11.27 g
Further explanation
A reaction coefficient is a number in the chemical formula of a substance involved in the reaction equation. The reaction coefficient is useful for equalizing reagents and products.
The reaction between Butanoic acid-C₄H₈O₂ and an excess Ethanol-C₂H₅OH to produce ethyl butyrate-C₆H₁₂O₂ :
C₄H₈O₂+C₂H₅OH⇒C₆H₁₂O₂+H₂O
mass Butanoic acid- C₄H₈O₂= 8.55 g, so mol C₄H₈O₂ (MW=88,11 g/mol) :
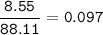

For 100% yield ⇒ actual=theoretical
Because Ethanol as an excess, so Butanoic acid as a limiting reactant and mol Ethyl butyrate based on mol Butanoic acid
From equation, mol ratio of mol C₆H₁₂O₂ : mol C₄H₈O₂ = 1 : 1, so : mol C₆H₁₂O₂=mol C₄H₈O₂= 0.097
mass C₆H₁₂O₂(MW=116.16 g/mol)