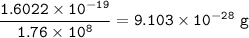The mass of one electron : 9.103 x 10⁻²⁸ g
Further explanation
Electrons are a part of the atomic nucleus particles (including protons and neutrons), and are negatively charged (-1)
An electron has the charge of -1.6022 x 10⁻¹⁹C
1 g = -1.76 x 10⁸ C
so the mass of one electron :