Answer:
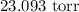
Step-by-step explanation:
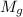 = Molar mass of glucose = 180.2 g/mol
= Molar mass of glucose = 180.2 g/mol
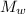 = Molar mass of water = 18 g/mol
= Molar mass of water = 18 g/mol
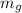 = Mass of glucose = 76.6 g
= Mass of glucose = 76.6 g
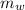 = Mass of water =
= Mass of water =
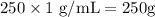
 = Vapor pressure of pure water at 25°C = 23.8 torr
= Vapor pressure of pure water at 25°C = 23.8 torr
The mole fraction of glucose is
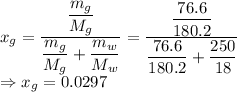
Mole fraction of the solute would be
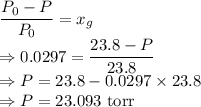
The vapor pressure of the solution is
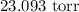 .
.