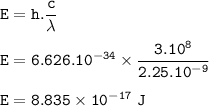The energy of a wave : 8.835.10⁻¹⁷ J
Further explanation
The energy in one photon can be formulated as
E = h. f
Where
h = Planck's constant (6.626.10⁻³⁴ Js)
f = Frequency of electromagnetic waves
f = c / λ
c = speed of light
= 3,10⁸ m/s
λ = wavelength
λ = wavelength of a wave = 2.25 nm = 2.25 x 10⁻⁹m