Answer:
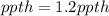
Step-by-step explanation:
Hello!
In this case, since the parts per thousand of a solute in a solute is defined in terms of its grams per kilogram of solution (unlike parts per million which are mg/kg) we can mathematically represent it via:
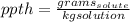
Now, since the whole human body acts as the solution and the chlorine in the form of ions as the solute, the required ppth turns out:
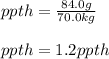
Best regards!