Hey There!
_____________________________________
Answer:
Solution:
a liquid mixture in which the the solute which is in a smaller proportion is uniformly distributed within the the solvent which is the major component is called a solution.
_____________________________________
Option A
Stirring the substance together vigorously:
It increases the rate of reaction, because when we stir the solute in the solution, the mixed solute particles are distributed more further than when they are left stationary, making the environment (which surrounds the solute) less concentrated by the dissolved solute thus the rate increases and more solute is mixed.
Option B
Dissolving the reacting substance in water:
No, A reacting substances in water are those which can react with water that are Ionic metal. Water is made of ions
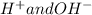 . Substances(solute), they mix or dissolve more because Solutes ions interact with the ions of a water(solvent), thus breaking the bonds between the solute and then ions of solute form bond with the ions of water. Thus the rate increases in water.
. Substances(solute), they mix or dissolve more because Solutes ions interact with the ions of a water(solvent), thus breaking the bonds between the solute and then ions of solute form bond with the ions of water. Thus the rate increases in water.
Option C
Increasing the temperature increases the rate of reaction:
No, because by the increase in temperature, the kinetic energy of more molecules of a substance increase which results in reaching the threshold energy(minimum energy required by the molecules to change into product) of more molecules. So less molecules are left with low energy.
Option D
Decreasing the temperature decrease the rate of reaction.
Yes, the rate of reaction decreases because the molecules are slower and collide less. Less molecules make the effective collisions due to which the kinetic energy of molecules is less, so less molecules make up to the threshold energy. So decrease in temperature lowers the rate of the reaction.
_____________________________________
Best Regards,
'Borz'