Answer:
0.805 M.
Step-by-step explanation:
Hello!
In this case, since the molarity of a solution is computing by dividing the moles of solute over the volume of solution in liters (M=n/V), for 15.0 g of potassium chloride (74.55 g/mol) we compute the corresponding moles:
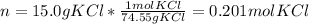
Next, since the volume is 0.2500 in liters, the molarity turns out:
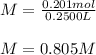
Best regards!