Answer:
Two-electron.
Step-by-step explanation:
Hello!
In this case, since the half-reaction by which chlorine is reduced to aqueous chloride ion is:
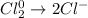
We can see each chlorine undergo a decrease of electrons by 1, it means that, since there are two chlorine atoms undergoing an electron decrease, we write:
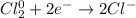
Which means this is a two-electron process.
Best regards!