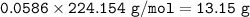Zn as a limiting reactant
N₂ as an excess reactant
Theoretical Yield : 13.15 g
Further explanation
Reaction
3 Zn + N₂ ⇒ Zn₃N₂
Ar Zn : 65,38 g/mol
Ar N₂ : 28.0134 g/mol
mol Zn
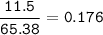
mol N₂
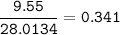
limiting reactant : the smaller ratio(mol:coefficient)
mol ratio Zn : N₂ :
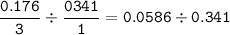
Zn as a limiting reactant(smaller ratio)
N₂ as an excess reactant
mol Zn₃N₂ :
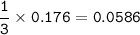
mass of Zn₃N₂ (Theoretical Yield) :