Answer:-Barium(Ba)
Step-by-step explanation:-
Electro negativity means the ability to attract ans take electron.(Highest in Fluorine)
- The halogens has highest EN than other families as they need only 1 electron to complete octet.
- Alkaline earth metals such as Be,Mg,Ca,Sc,Ba,Ra.
- They have 2 electrons in their valence shell but .
If we see their Electronic Configuration
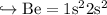
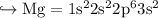
And so on
- The s subshell is completely filled (Duplet)
- So they are more stable .
- So they less attract electron as they has less EN