Answer:
39.9 L
Step-by-step explanation:
Use PV = nRT to solve this problem.
Substitute the values known.
P is pressure, which is 202 kPa, V is volume, which we do not know, n is moles, which is 3, R is 8.314, and T is temperature, which is 323K.
Set up your equation as follows
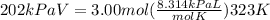
Solve for V, multiply the right side of the equation and divide by the pressure, 202 kPa.
This results in Volume being 39.9 L