Answer:
92.46 g
Step-by-step explanation:
The reaction is
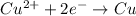
I = Current = 19.5 A
t = 4 hours =
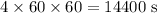
F = Faraday constant = 96485.33 C/mol
Molar mass of copper = 63.546 g/mol
Charge is given by
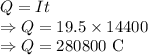
Moles of electrons is given by
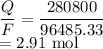
Moles of copper is
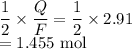
Mass of copper would be
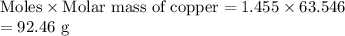
The mass of copper electroplated is 92.46 g.