Answer:
8.15 x 10¹⁴Hz
Step-by-step explanation:
Given parameters:
Energy of photon = 5.4 x 10⁻¹⁹J
h = 6.626 x 10⁻³⁴J.s
Unknown:
Frequency of the photon = ?
Solution:
The energy of a photon is given as;
E = hf
h is the Planck's constant
f is the frequency
f =
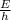 =
=
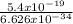 = 8.15 x 10¹⁴Hz
= 8.15 x 10¹⁴Hz