Answer:
0.4
Step-by-step explanation:
Given parameters:
Mass of NaCl = 0.564g
Mass of KCl = 1.52g
Mass of LiCl = 0.857g
Unknown:
Mole fraction of KCl = ?
Solution:
First, find the number of moles of the given species;
Number of moles =

Molar mass of KCl = 39 + 35.5 = 74.5g/mol
Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Molar mass of LiCl = 7 + 35.5 = 42.5g/mol
Number of moles of KCl =
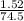 = 0.02mol
= 0.02mol
Number of moles of NaCl =
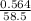 = 0.0096mol
= 0.0096mol
Number of moles of LiCl =
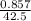 = 0.02mol
= 0.02mol
Sum of moles = 0.02mol + 0.0096mol + 0.02mol = 0.0496mol
Mole fraction of KCl =
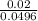 = 0.4
= 0.4