Answer:
2Fe(NO₃)₃ + 3MgO → Fe₂O₃ + 3Mg(NO₃)₂
Step-by-step explanation:
Given chemical species:
Reactants: Iron(III) nitrate
Magnesium oxide
Products: Iron (III) oxide
Magnesium nitrate
The chemical symbols:
Fe(NO₃)₃ + MgO → Fe₂O₃ + Mg(NO₃)₂
The reaction of this type is called a double displacement reaction.
Now let us ensure that it is balanced;
aFe(NO₃)₃ + bMgO → cFe₂O₃ + dMg(NO₃)₂
Conserving Fe;
a = 2c
Conserving N;
3a = 2d
Conserving O
9a + b = 3c + 6d
Conserving Mg
b = d
let a = 1, c =
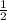 , d =
, d =
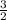 , b =
, b =
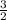
multiply through by 2;
a = 2, c = 1, d = 3 and b = 3
2Fe(NO₃)₃ + 3MgO → Fe₂O₃ + 3Mg(NO₃)₂