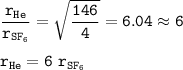rate He = 6 x rate SF₆
Further explanation
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
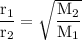
or
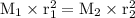
M He = 4 g/mol
M SF₆ = 146 g/mol