It is likely to receive one electron from another element's atom during the creation of a bond, based on the electron configuration. The arrangement of electrons in energy levels surrounding an atomic nucleus is termed electronic configuration, or electronic structure.
Fluorine's electron configuration is
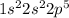
Because fluorine contains seven valence electrons, it is most likely to acquire one to create a 1-charged ion. To complete its octet, it will require one more electron. As a result, the oxidation number is 1, indicating that when fluorine interacts with another atom to form a more stable combination, it will acquire or share one electron. As a result, it is likely to acquire one electron from an atom of another element during the creation of a bond, based on the electron configuration.