Answer:
Methane: 1
Ethane: 2
Ethene: 2
Pentane: 5
Propene: 3
Hexane: 6
Ethyne: 2
Propane: 3
Hexane: 7
Octane: 8
Decane: 10
Butyne: 4
Butane: 4
Propyne: 3
Butene: 4
Step-by-step explanation:
Use the chemical formula of the compounds:
Methane ⇒
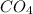 Hexane ⇒
Hexane ⇒
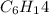 Decane ⇒
Decane ⇒
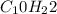
Ethane ⇒
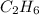 Ethyne ⇒
Ethyne ⇒
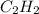 Butyne ⇒
Butyne ⇒
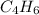
Ethene ⇒
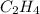 Propane ⇒
Propane ⇒
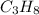 Butane ⇒
Butane ⇒
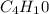
Pentane ⇒
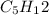 Hexane ⇒
Hexane ⇒
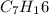 Propyne ⇒
Propyne ⇒
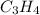
Propene ⇒
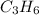 Octane ⇒
Octane ⇒
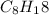 Butene ⇒
Butene ⇒
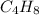
In the chemical formulas above, each formula has things known as coefficients (elements) and subscripts (atoms).
So to further explain, take methane as an example, there is a "C" at the beginning. C is capital as it is a pure substance and is a element. This also means there is 1 carbon atom in methane while there is 4 oxygen atoms. But to explain further, let's review definitions:
What is a compound in chemistry
A pure substance composed of two or more elements composed of two or more elements.
What is a pure substance in chemistry
A pure substance is a single element or compound, not mixed with any other substance
What is a element in chemistry
One of a class of substances that cannot be separated into simpler substances by chemical means.
What is a chemical formula (Alternative name for molecular formula)
A chemical formula that indicates the kinds of atoms and the number of each kind in a molecule of a compound.
So after reviewing let's find the carbon atoms.
The subscripts in each chemical formula that are to the right to the "C" are the amount of carbon atoms. If a capital letter does not have a subscript, it is assumed to have 1 atom only.
Your answers can be found above.