Answer:
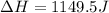
Step-by-step explanation:
Hello!
In this case, since the calorimeter contains a solution for which the specific heat is 4.18 J/g°C, which is equal to that of the water, we can also notice that the density is 1.00 g/mL and therefore the present mass is 250 g. In such a way, we compute the enthalpy change as shown below:
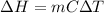
Thus, by plugging in the mass, specific heat and temperature change, we obtain:
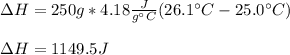
Best regards!