Answer:
0.36g
Step-by-step explanation:
Given parameters:
Volume of N₂H₄ = 350mL = 0.35dm³
Temperature of container = 50°C = 50 + 273 = 323K
Pressure = 885torr;
760torr = 1atm
885torr =
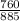 = 0.86atm
= 0.86atm
Unknown:
Mass of the compound = ?
Solution:
The mass of the compound can be derived using the expression below;
Mass = number of moles x molar mass
molar mass of N₂H₄ = 2(14) + 4(1) = 32g/mol
To find the number of moles;
PV = nRT
n =
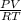
P is the pressure
V is the volume
R is the gas constant = 0.082atmdm³mol⁻¹K⁻¹
T is the temperature
n =
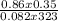 = 0.01mole
= 0.01mole
So,
Mass = 0.01 x 32 = 0.36g