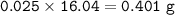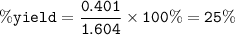The percentage yield of the reaction : 25%
Further explanation
C₂H₃NaO₂(CH₃COONa)- Sodium ethanoate(MW=82,0343 g/mol)
Reaction
CH₃COONa + NaOH⇒CH₄+Na₂CO₃
mol CH₃COONa :
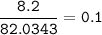
mol CH₄=mol CH₃COONa = 0.1
mass CH₄ (MW=16.04 g/mol) :
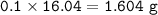 ⇒ theoretical
⇒ theoretical
mol of 560 cm³(0.56 L) of methane (⇒1 mol = 22.4 L at STP) :
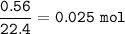
mass CH₄ :