Answer:
0.065M
Step-by-step explanation:
The reaction expression is given as:
HCl + KOH → KCl + H₂O
Given parameters:
Volume of KOH = 25.81mL = 0.02581L
Molarity of KOH = 0.125M
Volume of HCl = 50mL = 0.05L
Unknown:
Molarity of the acid = ?
Solution:
To find the molarity of the acid, HCl, let us use the mole concept.
First, find the number of moles of the base, KOH;
Number of moles of KOH = molarity of KOH x volume of KOH
Number of moles of KOH = 0.125 x 0.0258 = 0.003225mol
From the balanced reaction equation:
1 mole of KOH reacted with 1 mole of HCl
0.003225mol of KOH will react with 0.003255mole of HCl
Molarity of HCl =
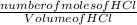
Molarity of HCl =
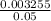 = 0.065M
= 0.065M