Answer:
0.07mol/kg
Step-by-step explanation:
Given parameters:
Mass of NaCl solution = 120g
Mass of water = 30kg
Unknown:
Molality of the solution =?
Solution:
The molality of a solution is the number of moles of the solute per mass of the solution.
Molality =
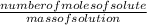
Number of moles of NaCl =

Molar mass of NaCl = 23 + 35.5 = 58.5g/mol
Number of moles of NaCl =
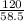 = 2.05mole
= 2.05mole
So;
molality =
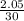 = 0.07mol/kg
= 0.07mol/kg