Answer:
37.3g
Step-by-step explanation:
Formula of the compound:
FeCl₃
Number of chloride ions = 4.13 x 10²³ ions
First find the number of moles in this ions;
6.02 x 10²³ ions are contained in 1 mole of a substance
4.13 x 10²³ ions contains
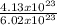 = 0.69mole chloride ions
= 0.69mole chloride ions
Now;
3 mole of chloride ions are contained in 1 mole of FeCl₃
0.69 mole of chloride ions will contain
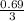 = 0.23mole of FeCl₃
= 0.23mole of FeCl₃
To find the mass;
Mass of FeCl₃ = number of moles x molar mass
molar mass of FeCl₃ = 55.8 + 3(35.5) = 162.3g/mol
Mass of FeCl₃ = 0.23 x 162.3 = 37.3g