Answer:
19.6 moles of aluminum sulfate
Step-by-step explanation:
Given parameters:
Number of sulfur atoms = 3.54 x 10²⁵ atoms
Unknown:
Number of moles of aluminum sulfate = ?
Solution:
Aluminum sulfate is expressed as;
Al₂(SO₄)₃
1 mole of Aluminum sulfate contains 3 moles of sulfur.
6.02 x 10²³ atoms are found in 1 mole of any substance;
3.54 x 10²⁵ sulfur atoms will be found in
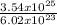 = 58.8moles of sulfur
= 58.8moles of sulfur
1 mole of Aluminum sulfate contains 3 moles of sulfur.
or ;
3 moles of sulfur is contained in 1 mole of aluminum sulfate
58.8moles of sulfur will contain
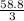 moles of aluminum sulfate
moles of aluminum sulfate
= 19.6 moles of aluminum sulfate